Experience
Program Achievements
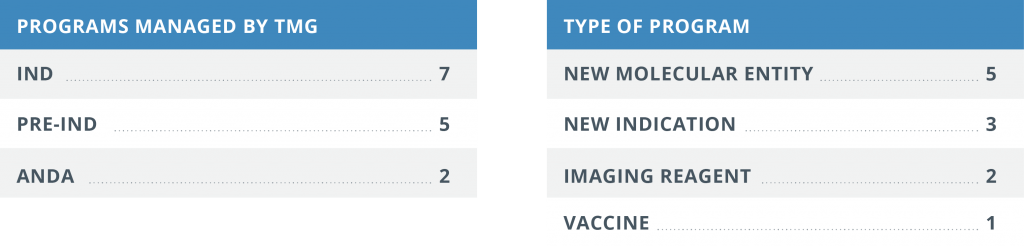
Since its conception, the Translational Medicine Group has successfully managed fourteen different therapeutic development programs ranging from pre-INDs through ANDAs for new drug candidates, repurposed drugs and biologics.
Clinical Trial Experience
Since 2009, the Translational Medicine Group has managed over 18 ongoing and completed clinical trials ranging from first-in-human through phase 2 for different therapeutic areas including type 2 diabetes, recurrent respiratory papillomatosis, and anemia. The TMG is currently preparing to initiate a phase 3 clinical program for the type 2 diabetes NME.
- NME for Type 2 Diabetes Mellitus:
- 13 clinical trials completed
- First in man, single and multiple ascending dose, PK/PD studies
- Ascending-dose study in subjects withT2DM
- Mass balance study using 14C-labeled drug substance
- QT study
- Single, multicenter, national and international studies
- Phase 2b, 96 week multicenter, double-blind, controlled international study
- New Drug Indication for Recurrent Respiratory Papillomatosis:
- 6 month, phase 1, single center safety, tolerability and efficacy study of an oncology drug in patients with RRP
- NCE for Anemia:
- First in man single and multiple ascending doses PK/PD study
- Phase 2a, POC in anemia patients on hemodialysis
- Phase 2a, POC in patients with chronic kidney disease who are anemic
Product Portfolio
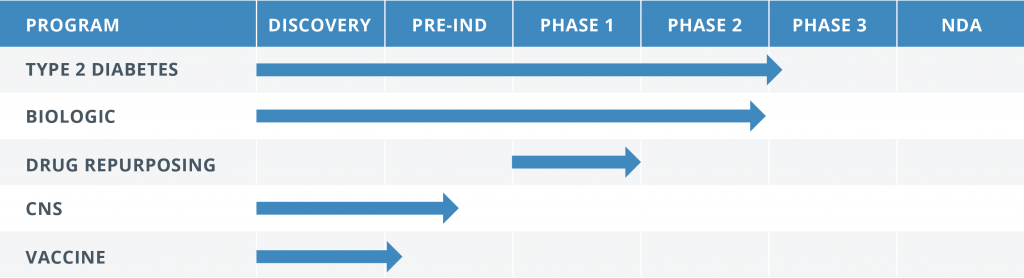
The Translational Medicine Group is currently managing the five different programs at various phases of regulatory development including new molecular entities, a repurposed drug and a biologic.
Case Study: NME for type 2 diabetes
- A management partnership was formed between the Translational Medicine Group and virtual biotech company for nonclinical, CMC and clinical development of NME for type 2 diabetes mellitus
- A medicinal chemistry campaign produced > 400 compounds
- Lead compound selected in late 2007
- Process development and manufacturing completed in 2008
- IND-enabling nonclinical studies completed 2009
- Since 2009, completed initial IND and 50 IND amendments
- Time from lead selection to IND filing: 62 weeks
- 44 nonclinical reports included in initial IND
- First subject enrolled 10 days after FDA review period concluded
- – To date, 79 nonclinical study reports and 126 clinical reports and supporting documents completed
- – Completed regulatory submissions in 5 countries (Including US) and treated over 650 human subjects
- Currently preparing for phase 3 studies
