
About
Welcome to the Translational Medicine Group. We were created in 2007 to accelerate the development of new therapeutics based on world-class research and clinical expertise available at MGH and Harvard Medical School. Our combined medical device and drug development expertise, strategic partnerships, translational core facilities and regulatory know-how enable cost effective and rapid delivery of breakthrough medical solutions. We are your partner in translational medicine.
Collaboration Isn’t Easy
Successful collaboration between industry and academia has proven to be difficult. Historically, cultural barriers, intellectual property concerns, conflict of interest and poor execution have prevented productive partnerships and prevented basic research findings from being tested in a clinical setting. MGH recognizes the need to change business as usual and has established the Translational Medicine Group to focus on building collaborative relationships with the biopharmaceutical industry to deliver breakthrough medical solutions to patients.
The New Paradigm
The discovery and development of new medicines is a long and expensive process. Taking a drug from early understanding of the disease at a molecular level, through development, and into the market may take 10 to 15 years. With a high rate of attrition and costs of up to $1.2 billion, the development of new drugs is a risky business. The Translational Medicine Group designs and conducts preclinical and early clinical trials that allow both the pharmacology of the drug as well as the biology of the target to inform the development process and quickly determine proof-of-concept.
We offer:
- Integrated translational drug development solutions for the biopharmaceutical industry
- Innovative and efficient drug & medical device development plans and project execution
- Industry experienced management team with proven track record of drug and medical device development and approval
- Timeline-driven, cost effective development of translational targets
- Streamlined contract negotiation, IP and IRB review processes
Tactical Focus
We combine the best practices of academia and industry and leverage the assets of the Boston biomedical community to create cross-functional teams with a thorough understanding of the drug development process to establish proof-of concept for new therapeutics.
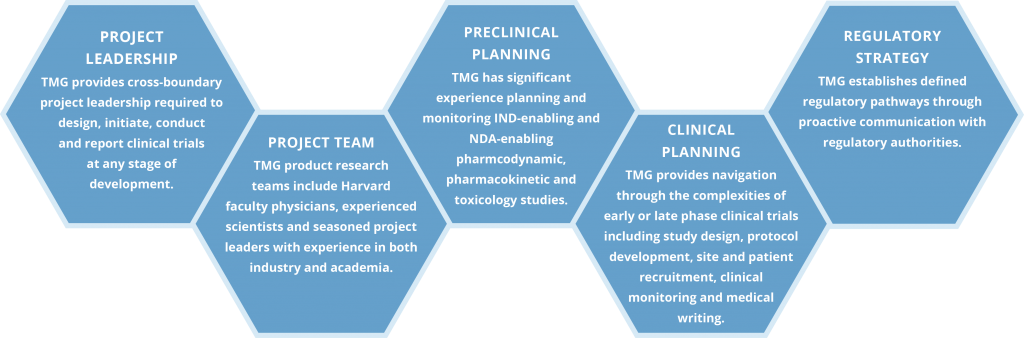
Limiting Risk in Translational Medicine
Significant numbers of new drug candidates fail in late stage clinical development due to lack of efficacy or unexpected toxicity, and at great expense. The Translational Medicine Group expedites proof-of-concept clinical testing for new molecular entities and cost effectively delivers drug candidates with decreased risk for phase 2b or 3 trials.

Key elements of our collaborative partnerships include:
- Motivated patient communities
- Biobank and state-of-the-art bioimaging techniques
- Clinical and scientific expertise
- Translational Research Center for proof-of-concept clinical trials
